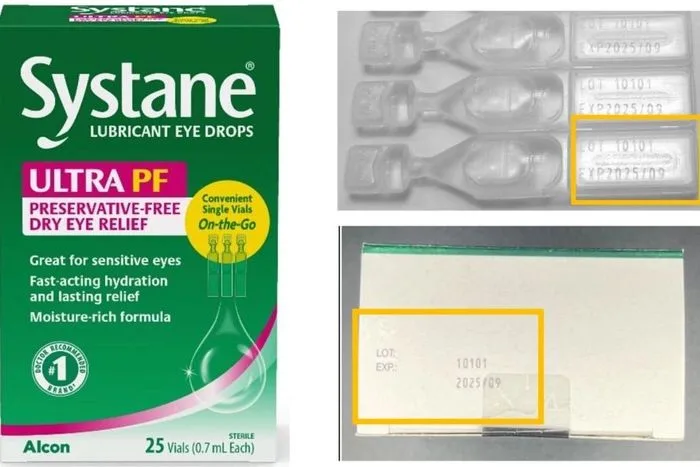
Eye Drops Recalled Due to Possible Fungal Contamination
By Naveen Athrappully An eye drop potentially contaminated with fungus is being withdrawn from the market nationwide because it poses health risks to users. The recalled item is one lot of “Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go” manufactured by Switzerland-based Alcon Laboratories, according to a Dec. 23 recall notice published by the U.S.
More Stories
US Trade Deficit Narrows Sharply to Lowest Level Since 2009
By Andrew Moran The U.S. trade deficit narrowed sharply in October 2025, falling to its lowest level in 16 years,...
Vance Announces New Assistant AG Position to Tackle Fraud Nationwide
By Travis Gillmore Vice President JD Vance announced the creation of a new assistant attorney general position to help investigate...
What to Know About the New ‘Upside Down’ Food Pyramid
By Lawrence Wilson The Department of Agriculture has turned the familiar food pyramid upside down, significantly revising the dietary guidelines...
Buying—Not Invading—Greenland Gains Momentum in Congress
By Nathan Worcester WASHINGTON—After capturing Venezuela’s now-former leader, Nicolás Maduro, the Trump administration has renewed its talk of acquiring Greenland,...
US Nuclear Energy Industry Poised to Power Up, but Bottlenecks Loom
By John Haughey Ten companies are developing “first mover” nuclear energy innovations in breakneck bids to achieve “criticality” by July...
China Steps Up Cyberattacks on Taiwan’s Critical Infrastructure, Testing Wartime Readiness
By Michael Zhuang China has dramatically escalated cyberattacks against Taiwan’s critical infrastructure, launching an average of 2.63 million attacks per...
